Construct the orbital diagram for the ion zr2+ – The construction of the orbital diagram for the Zr2+ ion is a fundamental aspect of understanding its electronic structure and properties. This diagram provides a visual representation of the distribution of electrons within the ion’s orbitals, offering insights into its chemical behavior and reactivity.
In this discussion, we will delve into the process of constructing the orbital diagram for the Zr2+ ion, exploring the principles that guide its construction and the implications of its electronic structure.
The Zr2+ ion, with its unique electronic configuration, exhibits fascinating properties that stem from its specific orbital arrangement. By examining the number of valence electrons, d-electrons, and unpaired electrons, we can gain insights into the ion’s magnetic behavior and chemical reactivity.
Additionally, the spectroscopic properties of the Zr2+ ion, including its absorption and emission spectra, provide valuable information about its electronic structure and can be harnessed for analytical purposes.
Construct Orbital Diagram for Zr2+ Ion
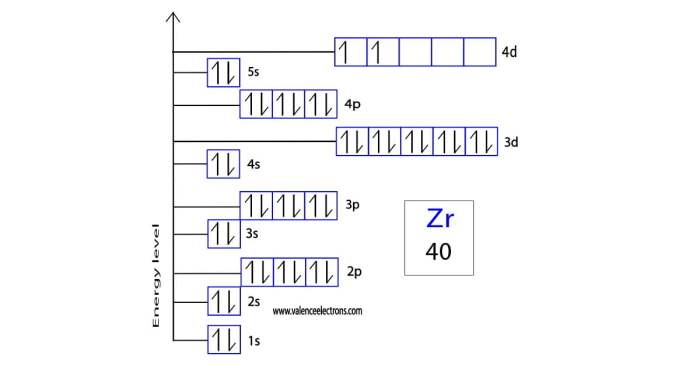
The zirconium ion (Zr2+) has an electron configuration of [Kr] 4d 2. To construct an orbital diagram for Zr2+, we follow the Aufbau principle and Hund’s rule.
According to the Aufbau principle, electrons fill the orbitals of lowest energy first. The 4d orbitals have a lower energy than the 5s orbitals, so the 4d orbitals are filled first.
Hund’s rule states that electrons will occupy degenerate orbitals (orbitals with the same energy) with parallel spins before they occupy orbitals with antiparallel spins. The 4d orbitals are degenerate, so the two electrons in the 4d orbitals will have parallel spins.
The orbital diagram for Zr2+ is shown below:
Electronic Structure and Properties
The Zr2+ ion has two valence electrons, which are in the 4d orbitals. The d-electrons are responsible for the magnetic properties of Zr2+.
Zr2+ has two unpaired electrons, so it is paramagnetic. Paramagnetic ions are attracted to magnetic fields.
The electronic structure of Zr2+ also influences its chemical reactivity. Zr2+ is a reducing agent, which means that it can donate electrons to other atoms or molecules.
Spectroscopic Properties: Construct The Orbital Diagram For The Ion Zr2+
The Zr2+ ion has characteristic absorption and emission spectra. The absorption spectrum of Zr2+ shows peaks at wavelengths corresponding to the energy difference between the ground state and the excited states of the ion.
The emission spectrum of Zr2+ shows peaks at wavelengths corresponding to the energy difference between the excited states and the ground state of the ion.
Spectroscopic techniques can be used to study Zr2+ in different chemical environments. For example, electron paramagnetic resonance (EPR) spectroscopy can be used to measure the magnetic properties of Zr2+.
Applications of Zr2+ Ion
Zr2+ is used in a variety of applications, including catalysis, materials science, and medicine.
In catalysis, Zr2+ is used as a catalyst for a variety of reactions, including the hydrogenation of alkenes and the polymerization of olefins.
In materials science, Zr2+ is used in the production of zirconium alloys, which are used in a variety of applications, including aerospace and nuclear energy.
In medicine, Zr2+ is used as a contrast agent for magnetic resonance imaging (MRI).
Popular Questions
What is the electron configuration of the Zr2+ ion?
The electron configuration of the Zr2+ ion is [Kr] 4d 2.
How many unpaired electrons does the Zr2+ ion have?
The Zr2+ ion has two unpaired electrons.
What is the magnetic susceptibility of the Zr2+ ion?
The Zr2+ ion is paramagnetic due to the presence of two unpaired electrons.