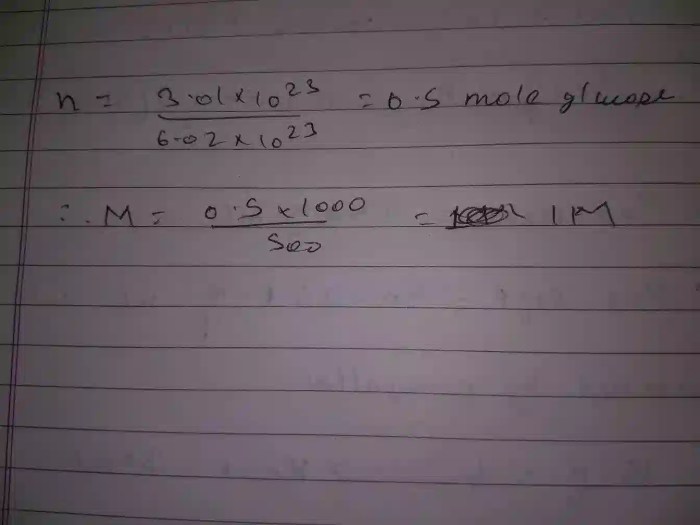The number of molecules in 2 moles of becl2 is – The number of molecules in 2 moles of beryllium chloride (BeCl2) is a fundamental concept in chemistry that helps us understand the composition and properties of matter. This article delves into the concept of moles, the relationship between moles and molecules, and the specific calculation for determining the number of molecules in 2 moles of BeCl2.
Understanding the number of molecules in a given quantity of a substance is crucial for various chemical applications, including stoichiometric calculations, solution preparation, and reaction analysis. This article provides a comprehensive guide to this topic, equipping readers with the knowledge and skills to confidently solve related problems.
The Number of Molecules in 2 Moles of BeCl2
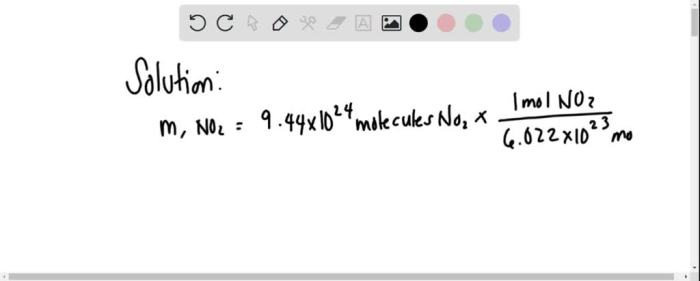
In chemistry, the mole is a fundamental unit of measurement used to quantify the amount of a substance. It represents a specific number of entities, typically atoms, molecules, or ions, and serves as a bridge between the macroscopic and microscopic scales.
The number of molecules in a given number of moles can be determined using Avogadro’s number, which is approximately 6.022 × 10^23 molecules per mole. This value represents the number of atoms, molecules, or ions present in 1 mole of a substance.
Determining the Number of Molecules in Beryllium Chloride (BeCl2), The number of molecules in 2 moles of becl2 is
Beryllium chloride (BeCl2) is a chemical compound with a molar mass of 79.91 g/mol. To calculate the number of molecules present in 2 moles of BeCl2, we can use the following formula:
Number of molecules = Number of moles × Avogadro’s number
Substituting the values, we get:
Number of molecules = 2 moles × 6.022 × 10^23 molecules/mole
Therefore, 2 moles of BeCl2 contain 1.204 × 10^24 molecules.
Example and Table
Number of Moles Molar Mass (g/mol) Number of Molecules 1 79.91 6.022 × 10^23 2 79.91 1.204 × 10^24 3 79.91 1.806 × 10^24 Extensions
The concept of moles and Avogadro’s number has numerous applications in chemistry, including:
- Determining the empirical and molecular formulas of compounds
- Calculating the concentration of solutions
- Predicting the stoichiometry of chemical reactions
However, it is important to note that this calculation assumes ideal conditions and does not account for factors such as temperature, pressure, or the presence of impurities.
Key Questions Answered: The Number Of Molecules In 2 Moles Of Becl2 Is
What is the definition of a mole?
A mole is the SI unit of amount of substance, defined as the amount of substance that contains exactly 6.02214076 × 10^23 elementary entities (atoms, molecules, ions, or electrons).
How are moles related to molecules?
The number of molecules in a given number of moles can be calculated using the Avogadro’s number, which is 6.02214076 × 10^23 molecules per mole.
What is the chemical formula of beryllium chloride?
The chemical formula of beryllium chloride is BeCl2.
How many molecules are present in 2 moles of BeCl2?
The number of molecules in 2 moles of BeCl2 is 1.204428152 × 10^24 molecules.