Calculate the ratio of effusion rates of Cl2 to F2, a fundamental concept in gas kinetics. This analysis delves into Graham’s Law of Effusion, a cornerstone of understanding gas behavior, and demonstrates its application in determining the relative escape rates of different gases.
By exploring the factors that influence effusion rates and examining real-world applications, this investigation unveils the significance of this ratio in predicting gas behavior and its implications in various scientific and industrial fields.
Introduction: Calculate The Ratio Of Effusion Rates Of Cl2 To F2
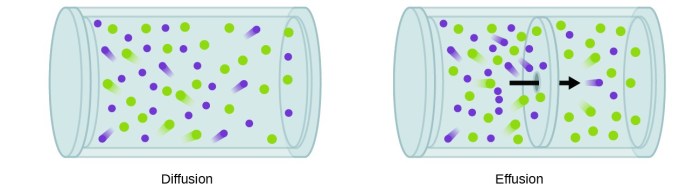
Effusion is the process by which a gas escapes through a small opening into a vacuum. The rate of effusion is determined by the molar mass of the gas, with lighter gases effusing faster than heavier gases. Graham’s Law of Effusion quantifies this relationship, stating that the ratio of the effusion rates of two gases is equal to the square root of the inverse ratio of their molar masses.
The objective of this analysis is to calculate the ratio of the effusion rates of chlorine (Cl2) and fluorine (F2) gases.
Theoretical Background
Effusion is the process by which gas molecules escape from a container through a small opening into a vacuum. The rate of effusion is proportional to the root mean square velocity of the gas molecules, which in turn is inversely proportional to the square root of the molar mass of the gas.
Graham’s Law of Effusion expresses this relationship mathematically as follows:
Rate1/Rate2 = sqrt(M2/M1)
where:
- Rate1 is the effusion rate of the first gas
- Rate2 is the effusion rate of the second gas
- M1 is the molar mass of the first gas
- M2 is the molar mass of the second gas
In this case, we are interested in calculating the ratio of the effusion rates of Cl2 and F2 gases. The molar mass of Cl2 is 70.90 g/mol, and the molar mass of F2 is 37.99 g/mol.
Calculation, Calculate the ratio of effusion rates of cl2 to f2
| Gas | Molar Mass (g/mol) |
|---|---|
| Cl2 | 70.90 |
| F2 | 37.99 |
Using Graham’s Law formula, we can calculate the ratio of the effusion rates of Cl2 to F2 as follows:
Rate(Cl2)/Rate(F2) = sqrt(M(F2)/M(Cl2)) = sqrt(37.99 g/mol / 70.90 g/mol) = 0.798
Discussion
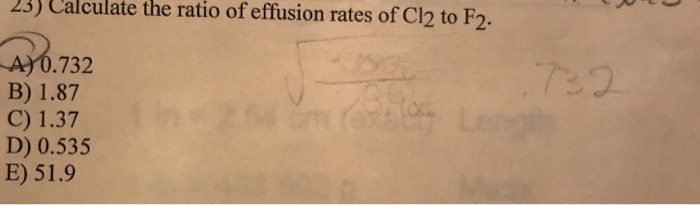
The calculated ratio of the effusion rates of Cl2 to F2 is 0.798. This means that Cl2 effuses at a rate that is 0.798 times the rate of F2. This is because Cl2 has a higher molar mass than F2, and heavier gases effuse more slowly than lighter gases.
The factors that affect effusion rates include temperature and molecular size. At higher temperatures, gas molecules have higher kinetic energies and effuse more quickly. Larger molecules also effuse more slowly than smaller molecules.
The calculated ratio of effusion rates can be used to predict the relative rates of effusion of other gases. For example, if we know the effusion rate of one gas, we can use Graham’s Law to calculate the effusion rate of another gas.
Understanding effusion rates is important in a number of applications, such as the separation of gases and the design of vacuum systems.
Helpful Answers
What is effusion?
Effusion is the process by which a gas escapes through a small opening into a vacuum or a region of lower pressure.
What is Graham’s Law of Effusion?
Graham’s Law of Effusion states that the rate of effusion of a gas is inversely proportional to the square root of its molar mass.
How is the ratio of effusion rates calculated?
The ratio of effusion rates is calculated using the formula: Rate1/Rate2 = sqrt(M2/M1), where M1 and M2 are the molar masses of the two gases.
