Which of the molecules and polyatomic ions cannot be adequately represented? This question opens the door to an intriguing exploration of the limitations of current scientific understanding. We delve into the criteria used to assess the adequacy of representation, uncovering the challenges posed by certain molecules and ions.
Alternative representations are examined, highlighting their advantages and disadvantages.
The implications of this topic extend beyond theoretical chemistry, reaching into fields such as materials science and biology. Understanding the limitations of representation enables researchers to identify areas where further investigation is crucial. This journey promises to shed light on the complexities of molecular and ionic structures, pushing the boundaries of scientific knowledge.
Molecules and Polyatomic Ions: Which Of The Molecules And Polyatomic Ions Cannot Be Adequately
Molecules are neutral entities composed of two or more atoms chemically bonded together. Polyatomic ions are charged species containing multiple atoms that behave as a single unit.
Criteria for Adequate Representation
Adequately representing a molecule or polyatomic ion requires considering its molecular structure, bonding, and charge distribution. Accurate representations should reflect the arrangement of atoms, the nature of the chemical bonds, and the distribution of electrons within the species.
Molecules and Polyatomic Ions that Cannot be Adequately Represented, Which of the molecules and polyatomic ions cannot be adequately
Certain molecules and polyatomic ions pose challenges for adequate representation due to their complex structures or bonding characteristics.
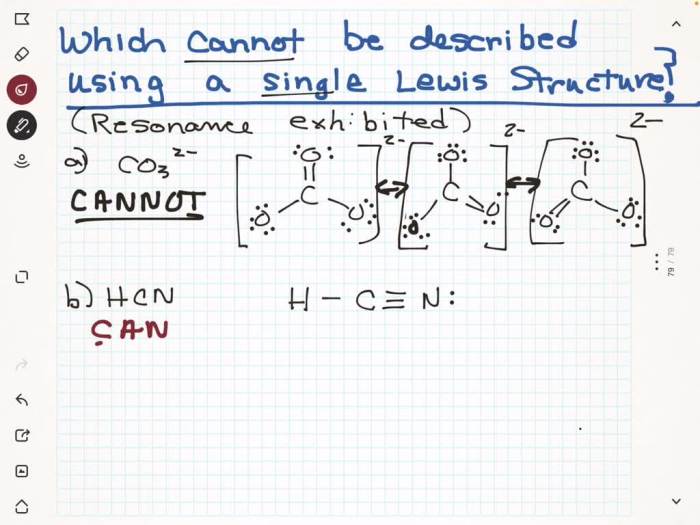
- Benzene:Its resonance structure, involving alternating double and single bonds, cannot be fully captured by traditional Lewis structures.
- Transition metal complexes:Their d-orbital interactions and variable oxidation states make them difficult to represent accurately using simple bonding models.
- Polyatomic ions with delocalized electrons:Ions like NO 3–and SO 42-have resonance structures and delocalized electrons, making their bonding difficult to describe using localized bonding models.
Alternative Representations
To overcome these challenges, alternative representations have been developed:
- Resonance structures:Depict multiple possible electron configurations for molecules like benzene, providing a more accurate representation of their bonding.
- Molecular orbital theory:Describes the electronic structure of molecules using wave functions, providing insights into bonding and electron distribution.
- Density functional theory:Employs computational methods to determine the electron density distribution in molecules, offering detailed information about their bonding and properties.
Applications and Implications
Understanding the limitations of molecular representations has practical implications:
- Chemistry:Accurate representations are crucial for predicting molecular properties, reactivity, and reaction mechanisms.
- Materials science:Understanding bonding in complex materials like transition metal oxides is essential for designing materials with desired properties.
- Biology:Accurate representations of biomolecules, such as proteins and nucleic acids, are vital for understanding their structure and function.
Clarifying Questions
Why is it important to adequately represent molecules and polyatomic ions?
Accurate representation is essential for understanding their properties, predicting their behavior, and designing materials and processes that rely on them.
What are the challenges in representing certain molecules and polyatomic ions?
Challenges arise due to factors such as complex molecular structures, strong correlations between electrons, and the need for high computational accuracy.
How do alternative representations address these challenges?
Alternative representations, such as density functional theory and molecular dynamics simulations, provide more accurate descriptions of molecular and ionic systems, albeit with their own limitations.