Cracking the periodic table code pogil – Embarking on a journey to decipher the enigmatic code of the periodic table, this discourse unveils the profound patterns and relationships that govern the chemical world. By delving into the periodic table’s intricate structure and unraveling the periodic trends, we embark on a quest to unlock the secrets of matter and its behavior.
Through a systematic exploration of atomic properties, electron configurations, and chemical reactivity, we uncover the underlying principles that orchestrate the symphony of elements. The periodic table emerges as a roadmap, guiding our understanding of the chemical landscape and empowering us to predict and manipulate the properties of matter.
1. Introduction to Cracking the Periodic Table Code POGIL
The Process Oriented Guided Inquiry Learning (POGIL) activity on “Cracking the Periodic Table Code” is a guided inquiry-based learning experience designed to enhance students’ understanding of the periodic table and its applications.
The activity aims to help students develop their problem-solving skills, critical thinking abilities, and understanding of chemical principles by engaging them in hands-on exploration and analysis of the periodic table.
2. Key Concepts in the Periodic Table
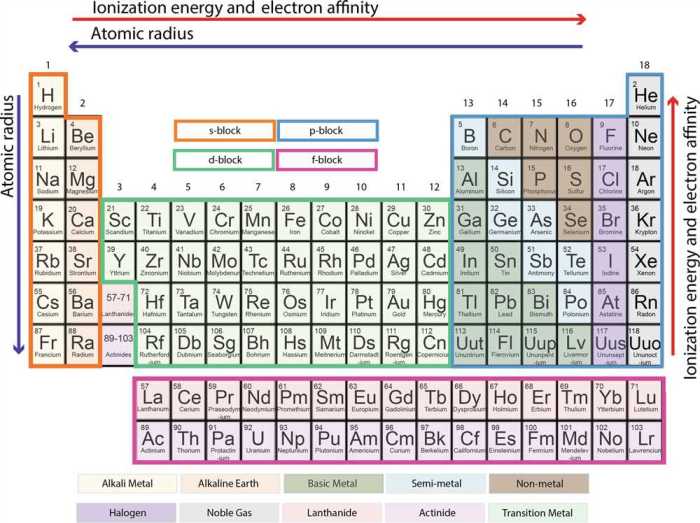
The periodic table is a tabular arrangement of chemical elements, organized based on their atomic number, electron configuration, and recurring chemical properties.
Basic Structure
- Elements are arranged in 18 vertical columns, called groups, and 7 horizontal rows, called periods.
- Groups represent elements with similar chemical properties due to having the same number of valence electrons.
- Periods represent elements with increasing atomic number and decreasing atomic radius.
Periodic Trends
- Atomic number: Increases from left to right across a period and from top to bottom within a group.
- Atomic mass: Generally increases from left to right across a period and from top to bottom within a group.
- Electronegativity: Generally increases from left to right across a period and from bottom to top within a group.
3. Patterns and Relationships in the Periodic Table
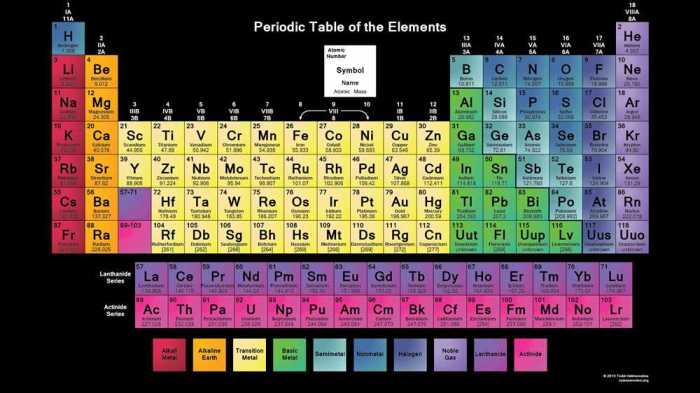
The periodic table exhibits distinct patterns and relationships that govern the chemical reactivity and properties of elements.
Chemical Reactivity
- Elements in the same group tend to have similar chemical reactivity due to their shared valence electron configuration.
- Metals are generally found on the left side of the table and are highly reactive, while nonmetals are found on the right side and are less reactive.
Electron Configuration and Chemical Properties, Cracking the periodic table code pogil
- The electron configuration of an element determines its chemical properties.
- Elements with similar electron configurations tend to have similar chemical properties.
Ionization Energy and Electron Affinity
- Ionization energy is the energy required to remove an electron from an atom.
- Ionization energy generally increases from left to right across a period and decreases from top to bottom within a group.
- Electron affinity is the energy change when an atom gains an electron.
- Electron affinity generally increases from left to right across a period and from top to bottom within a group.
4. Applications of the Periodic Table
The periodic table is a valuable tool for predicting and understanding the chemical properties of elements and compounds.
Predicting Chemical Properties
- The periodic table allows scientists to predict the chemical properties of an element based on its position in the table.
- For example, elements in the same group tend to have similar reactivity, so the reactivity of an unknown element can be predicted based on its group membership.
Classifying Elements and Compounds
- The periodic table helps classify elements into categories such as metals, nonmetals, metalloids, and noble gases.
- Compounds can also be classified based on the elements they contain.
Applications in Science
- The periodic table is used in various fields of science, including chemistry, physics, biology, and materials science.
- It provides insights into the structure, properties, and behavior of atoms and molecules.
5. Extensions and Challenges: Cracking The Periodic Table Code Pogil
While the periodic table is a powerful tool, it also has limitations and areas for ongoing research.
Limitations
- The periodic table does not fully explain the behavior of transition metals and lanthanides.
- It does not account for the effects of electron correlation and relativistic effects on atomic properties.
Current Research and Advancements
- Researchers are exploring new ways to modify and extend the periodic table to account for these limitations.
- This includes developing new theoretical models and experimental techniques to probe the electronic structure of atoms and molecules.
Suggestions for Further Investigation
- Students can explore the history of the periodic table and the contributions of scientists like Dmitri Mendeleev.
- They can also investigate the applications of the periodic table in different fields of science and technology.
Top FAQs
What is the purpose of the POGIL activity on cracking the periodic table code?
The POGIL activity aims to guide students in actively exploring the periodic table, uncovering its patterns and relationships, and developing a deeper understanding of chemical properties.
How does the periodic table help predict chemical properties?
The periodic table allows us to identify trends in atomic properties, such as electronegativity and ionization energy, which can be used to predict the chemical reactivity and behavior of elements.
What are the limitations of the periodic table?
While the periodic table provides a powerful framework for understanding chemical properties, it does not account for all chemical phenomena, such as the existence of isotopes and the complexities of chemical bonding.
